Types of psychoactive compounds
Society often misunderstands what drugs are. We all have a family member or a friend who says they are against drugs, but they consume alcohol, coffee, or smoke.
Likewise, the community blurred the narrow line between what is psychedelic or simply psychoactive. We have constant arguments on whether MDMA, Cannabis, or Ketamine might be psychedelic or not.
To some, the compounds become psychedelics based on the dosages. To others, the compounds fall into more scientific terminologies.
So, what exactly is a psychedelic, and where do we lay the line between what is a psychedelic or a psychoactive compound? What are the other psychoactive classes, and how can we distinguish them?
Aldous Huxley first suggested “phanerothyme,” from the Greek words for “to show” and “spirit,” and sent a rhyme: “To make this mundane world sublime, Take half a gram of phanerothyme.” To describe the word psychedelic, a term coined later by Osmond Humphrey from the Greek words psyche (for mind or soul) and deloun (for show), and suggested, “To fathom Hell or soar angelic/Just take a pinch of psychedelic.”

By following this terminology, the psychedelic community will have an endless debate on whether a compound is psychedelic or simply psychoactive. Since the terms spirit or soul are subjective, it is impossible to determine whether someone is showing their spirit or soul scientifically. One may be able to show the mind or soul by using, for instance, MDMA or Ketamine, and others might argue that these compounds are not psychedelic and belong to other categories.
Only science can determine the result of this debate. Unlike the terms spirit or soul, science can be objective. For this reason, science classifies various compounds by their chemical structure, effects, and pharmacological proprieties.
Without further ado, we present you the various groups that belong to psychoactive drugs:

Cannabinoids (Classical Cannabinoids, Carbazoles, Cyclohexyl-substituted phenols, Naphthoylindoles, the URB-class, and Benzoylindoles.)
Cannabinoids are substances found in Cannabis and compounds with a similar structure to those found in the Cannabis plant.

Experiences with Cannabinoids often resemble those present in the main psychoactive compound of Cannabis, d9-THC. Hence, users report feelings of relaxation, euphoria, and disinhibition.
Due to its effect and popularity, synthetic cannabinoids follow the chemical structure of d9-THC and are referred to as synthetic cannabinoids due to their pharmacological mechanism.
These compounds act primarily with the endocannabinoid system and its two specific G-protein coupled receptors: predominantly with the CB1 and less frequently with the CB2 receptors.
The endocannabinoid system plays a vital role in physiological functions like cognition, motor control, pain sensation, appetite, cardiovascular and respiratory performance, gastrointestinal motility, and immunoregulation.
Examples of Cannabinoids:
-
- THC (tetrahidrocanabinol)
-
- CBD (Cannabidiol)
-
- CBN (Cannabinol)
-
- CBG (Cannabigerol)
-
- CBC (Cannabichromene)
-
- THCV (Tetrahydrocannabivarin)
-
- THCP (Tetrahydrocannabiphorol)
-
- AM-694
-
- RCS-4
-
- CP-47,497
-
- JWH-018
-
- RCS-8
Depressants (Sedatives, Hypnotics)
Depressants are substances that decrease the activity in the brain. These compounds lower neurotransmission levels, thus depressing (reducing) arousal/ stimulation in various neural sites.
Depressants work through various pharmacological mechanisms, but the most prominent include the facilitation of GABA or Opioid activity and the inhibition of glutamatergic or catecholaminergic activity.
Examples of Depressants:
-
- 2M2B
-
- Alcohol
-
- Amanita Muscaria
-
- Antipsychotics
-
- Barbiturates
-
- Benzodiazepines
-
- Clonazepam
-
- Gabapentinoids
-
- GHB
-
- Kava
-
- Parafluorofentanyl
-
- Thienodiazepines
-
- Xanax

Dissociatives/Anesthetics (Arylcyclohexylamine, Diarylethylamine)
Dissociatives have two main classes. Arylcyclohexylamines and Diaryethylamines.
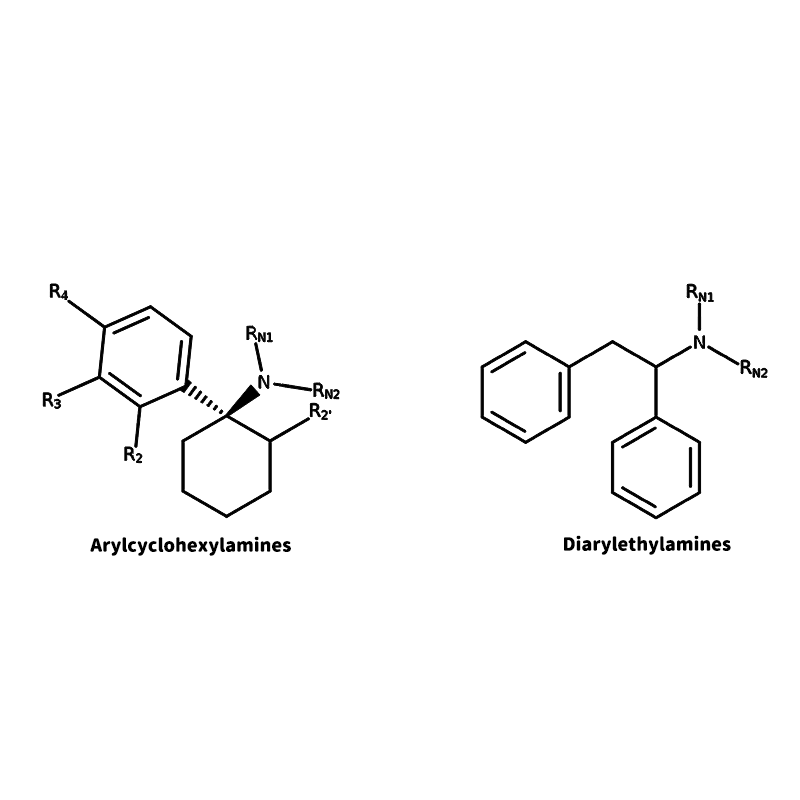
Both of these classes act as antagonists in the N-methyl-d-aspartate receptor (NMDAR), which has a role in synaptic plasticity and synapse formation, underlying memory, learning, and formation of neural networks during development in the central nervous system.

These compounds are sought after due to their sense of disconnection between thoughts, identity, memory, and consciousness, but also for their sensory and tactile distortions, euphoria, and depersonalization.
In addition, some compounds (PCP and its analogues) may also act in the serotonin receptors, which may explain some of the additional effects present in the experiences.
Examples of Arylcyclohexylamines:
-
- Ketamine
-
- PCP
-
- MXE
-
- 4-MeO-PCP
-
- 3-MeO-PCP
-
- 3-MeO-PCE
Examples of Diarylethylamines:
-
- MK-801
-
- MXP
-
- Ephenidine
-
- Fluorolintane
-
- NPDPA
Empathogens/Entactogens
Empathogens/Entactogens got their name for increasing a person’s empathy, benevolence towards others, and feeling socially accepted and connected.
These compounds release dopamine and serotonin in the brain.
Due to their properties, empathogens could be the perfect companions for therapists, as by administering such substances, they can delve into a patient’s issue and make the patient more open to talking about traumas or issues.
Examples of Empathogens/Entactogens:
-
- MDMA (ecstasy)
-
- MDA
-
- Mephedrone
-
- Ethylone
Opioids:
This category includes opiates, semi-synthetic opioids, and synthetic opioids.
Opiates are natural substances that originate from the opium poppy (Papaver somniferum)
Opioids interact with the G protein-coupled opioid receptors in the brain and spinal cord as partial to full agonists at mu, delta, and kappa opioid receptor subtypes, with selectivity for the mu-opioid receptor. Agonism at the mu-opioid receptors is responsible for the pharmacological effects of opioids, those being euphoria, analgesia, and respiratory depression, as well as the development and dependence.

Semi-synthetic and synthetic opioids bind to the same receptors as opiates and produce similar effects.
Examples of Opioids:
-
- Buprenorphine
-
- Codeine
-
- Heroin
-
- Methadone
-
- Opium
-
- Fentanyl
-
- Oxycodone
-
- Morphine
Stimulants (Natural and Synthetic Stimulants):
Usually, we divide stimulants into the following groups: Cathinones, Aminoindanes, Phenethylamines, Piperazines, and Tryptamines, of which synthetic cathinones are the largest group studied.

Synthetic cathinones replicate the effect of traditional stimulant controlled substances such as Cocaine, MDMA, and Amphetamines.
These compounds increase the synaptic availability of neurotransmitters, mainly dopamine, serotonin, and noradrenaline, to a lesser extent. Dopamine plays a role in motivation, arousal, and learning reward, whereas serotonin contributes to feeling a sense of emotional connectedness and mood.
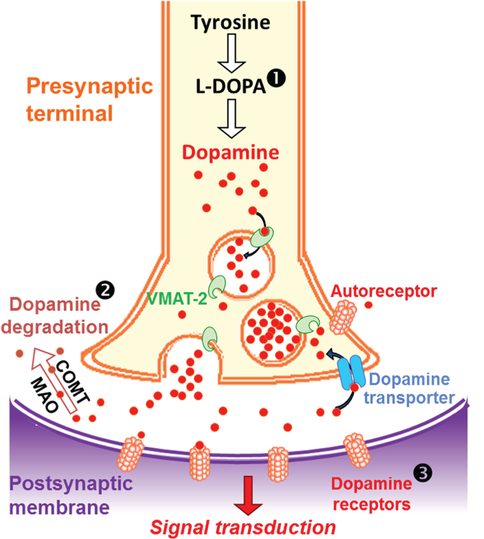
Stimulants act on dopamine and serotonin to different extents, accounting for their differing range of desired and unwanted effects. These include euphoria, increased empathy and compassion, a sense of inner peace and relaxation, enhanced self-confidence, sociability and libido, and boosted energy and alertness.
These compounds have a high addiction potential and can cause severe intoxications linked to cardiac and neurological complications, resulting in an increased number of fatalities.
Examples of Synthetic and Natural stimulants:
-
- Some examples of Synthetic and Natural stimulants:
-
- Amphetamines
-
- Betel Nut
-
- Caffeine
-
- Cocaine
-
- Methamphetamine – Ice
-
- Khat
-
- Nicotine
-
- Synthetic Cathinones: MDPV (3,4-methylenedioxypyrovalerone), 4-FMC (4-fluoromethcathinone), 4-MMC (4-methylmethcathinone)(mephedrone).
Psychedelics (Tryptamines, Lysergamides, and Phenethylamines):
Psychedelics share a common mechanism of 5-HT2A receptor modulation of serotonergic activity, although there is an increase in the role of the glutamatergic system, and some dissociative hallucinogens have activity at the κ opioid receptors.
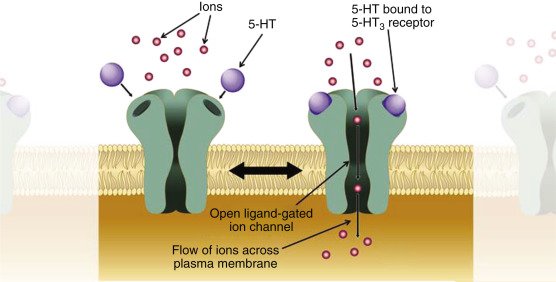
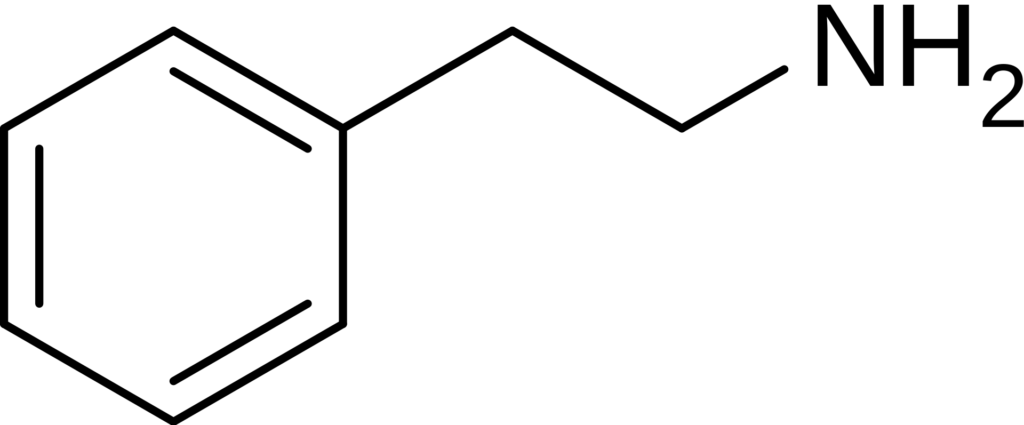
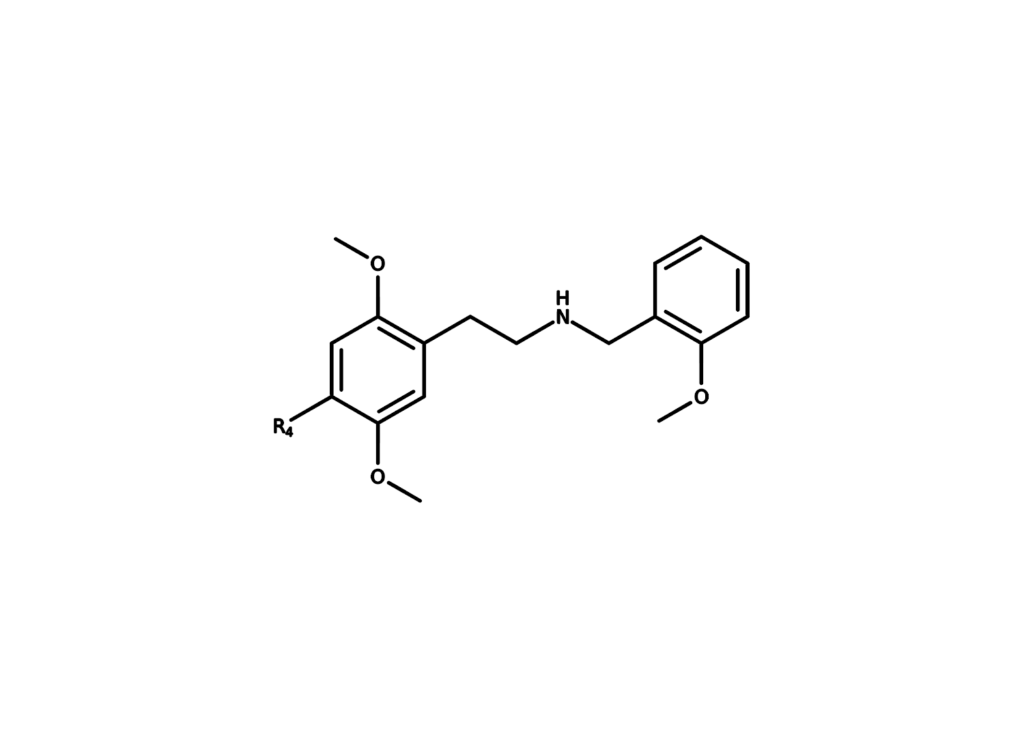
The largest group of synthetic psychedelics are the phenethylamine derivatives, which are 2,5-dimethoxyphenethylamines and contain a small lipophilic substituent at the 4-position, known as the 2C series since they possess two carbon atoms between the benzene ring and amino group synthesized by Alexander Shulgin. Further derivatives are mostly but not exclusively chemically modified at the phenyl ring. When a phenethylamine is modified with an N-benzylmethoxy (‘NBOMe’) group, the resulting derivatives become more potent.
Phenethylamines interact mainly with cortical serotonin receptors, with the highest affinity for 5-HT2A receptors.
Meanwhile, NBOMe derivatives have a higher affinity for 5-HT2A and 5-HT2C receptors and a lower affinity to the 5-HT1A receptors compared to their 2C analogues.
Examples of Psychedelic Phenethylamines:
-
- 2C-B
-
- 2C-I
-
- Mescaline
-
- DME
-
- F-22
-
- TME
Examples of NBOMe derivatives:
-
- 25I-NBOMe
-
- 25I-NBF
-
- 25I-NBOH
Tryptamines are a group of monoamine alkaloids synthesized through decarboxylation of tryptophan. They possess an indole ring structure, a bicyclic combination of a benzene ring and a pyrrole ring, with an amino group attached to a 2-carbon side chain.
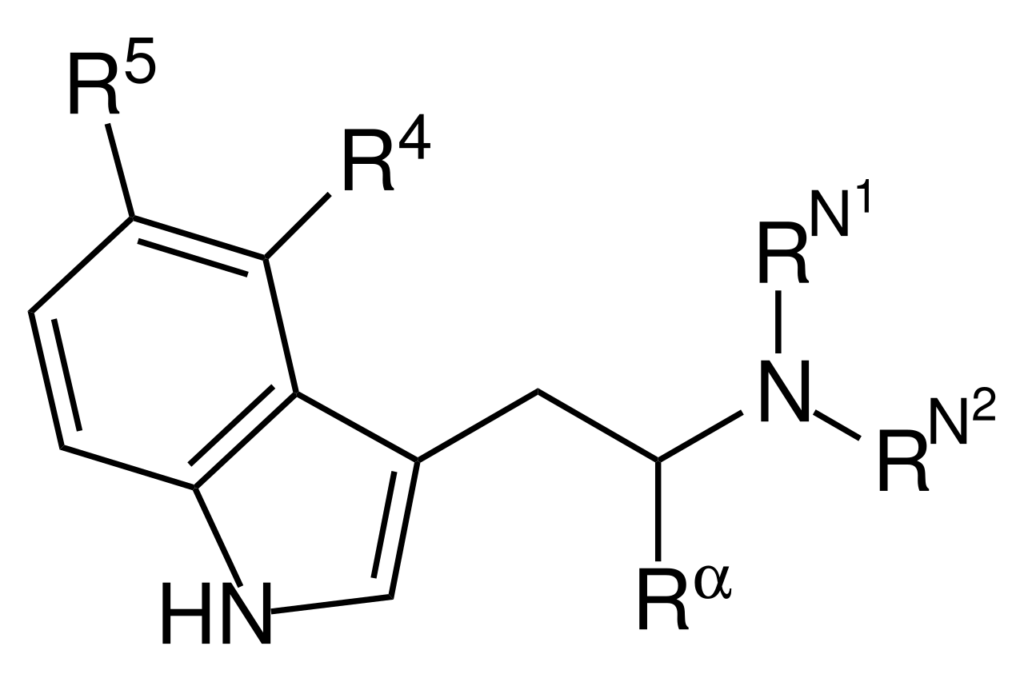
Tryptamine derivatives have an affinity for the 5-HT1A, 5-HT2A, and 5-HT2C receptors and can inhibit reuptake and increase the release of serotonin.
Examples of Psychedelic Tryptamines:
-
- DMT
-
- 5-MeO-DMT
-
- DiPT
-
- AMT
-
- Psilocybin
-
- DET
-
- 4-HO-αMT
-
- 5-MeO-DALT
-
- MiPT
Lysergamides are synthetic derivatives of the ergot alkaloid Lysergic Acid Diethylamide (LSD).

Lysergamides activate both 5-HT2A and 5-HT1A receprots. By activating the 5-HT2A receptors it causes glutamate release and activation of AMPA glutaminergic receptors, thus increasing cortical activity and information processing.
Examples of Lysergamides:
- LSA
- LSB
- PRO-LAD
- ALD-52
- 1P-LSD
- AL-LAD
We hope this list of various classes helps you understand the difference between the extensive research of psychoactive compounds, whether it is during your scientific journey or the journeys through the provinces of the mind!








