Slow releasing Ketamine tablets might be the future for the treatment of severe depression
Ketamine is typically known for its veterinary use as a horse tranquilizer, clinically as an anesthetic, and in nightclubs as a party drug. Recently, the notion of Ketamine has shifted from what we used to know, and the drug became seen as a potential tool for the treatment of various mental illnesses such as severe depression, resistant depression, PTSD, end-of-life distress, chronic pain, drug/alcohol problems, in between others.
It is essential to know that Ketamine typically consists of a racemic mixture of two enantiomers, R-ketamine (Arketamine)) and S-Ketamine (or Esketamine). To those not familiar with chemical terminologies, imagine your hands. They are not superimposable if you try to put them on top of each other. Instead, they are mirror images. The same happens with enantiomers. They are mirrored images of a molecule.
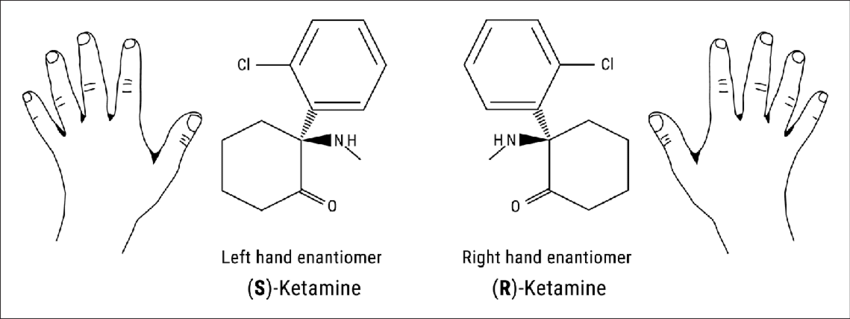
At the time of writing this article, two versions of therapy coexisted. The IV Ketamine treatment used Ketalar (Ketamine hydrochloride; the racemic mixture of the R- and S- Ketamine) and Johnson & Johnson’s nasal spray Spravato (S-Ketamine or Esketamine). Both of these products have their downside. Ketamine clinics that administer Ketalar are often far from users, and even with the resurgence of various clinics worldwide, sometimes patients need to travel from state to state during the treatment or even to different countries, making the experience inaccessible for plenty of patients.
In the case of Esketamine, being a nasal spray opened up various possibilities for not needing to travel around for a Ketamine session. However, each canister costs about $800 plus the cost of the integration therapy, and they are not covered by insurance, thus making this method also inaccessible for plenty of patients.
In addition, there is a constant ongoing discussion about whether S-ketamine is the compound to go to, considering R-ketamine (Arketamine) seems a more fitting therapeutical compound than S-ketamine.
The reasoning behind this discussion lies in the shift in understanding of how the compound produces its antidepressant effects. Previously, it was recognized that the antidepressant effects of Ketamine could be due to the inhibition of NDMAR. This belief was the cause of Johnson & Johnson developing Esketamine, considering the compound has a higher affinity to the NMDAR than Arketamine.
Research published in the Neuropharmacology journal by Ji-chun Zhang, Wei Yao, and Kenji Hashimoto from Chiba University in Japan. Suggested that Arketamine (PCN-101) produced a more sustained antidepressant effect while causing fewer psychomotor and behavioral side effects at therapeutic doses and had a lower potential for abuse in comparison to S-Ketamine.
The researchers are trying to prove the superiority of Arketamine over Esketamine using animal models of depression. It is added that this study was successfully reproduced by other research teams.(Fukumoto et al., 2017; Rafalo-Ulinska and Palucha-Poniewiera, 2022; Zanos et al., 2016; Zhu et al., 2020). Collectively, it is likely that Arketamine would be a new rapid-acting antidepressant without the side effects of Esketamine.
Differences between the mechanisms of action of Arketamine and Esketamine:
Roles of the NMDAR* and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)
Esketamine:
-
- NMDAR Potency: Esketamine is more potent at blocking NMDARs than arketamine, which contributes significantly to its antidepressant effects (Yang et al., 2015, 2018b; Zhang et al., 2014, 2020).
-
- AMPAR Activation: AMPAR activation is essential for the antidepressant-like effects of Esketamine, as AMPAR antagonists can block its antidepressant effects in rodent models (Autry et al., 2011; Fukumoto et al., 2017; Li et al., 2010; Yang et al., 2015; Zanos et al., 2016).
Arketamine:
-
- NMDAR Potency: Arketamine is less potent at blocking NMDARs than Esketamine (Yang et al., 2015, 2018b; Zhang et al., 2014, 2020).
-
- AMPAR Activation: AMPAR activation contributes to the antidepressant-like effects of arketamine, but the role is less pronounced compared to esketamine (Autry et al., 2011; Fukumoto et al., 2017; Li et al., 2010; Yang et al., 2015; Zanos et al., 2016).
*NMDA receptor antagonists increase mature synaptic protein expression, promote synaptogenesis, increase the number, morphology, and activity of spine synapses, and reduce depressive-like behaviors in rodent models of depression
*There is an increasing body of evidence implicating a role for alpha-amino-3-hydroxy-5-methyl-4 isoxazoleproprionic acid (AMPA) receptors in major depression and the actions of antidepressant drugs. Alterations in AMPA receptors and other ionotropic glutamate receptors have been reported in depression and following antidepressant treatment.
Extracellular signal-regulated kinase (ERK)* and ERK-NRBP1-CREB-BDNF* signaling
Esketamine:
-
- ERK Signaling: Esketamine has a less pronounced role in activating ERK signaling than arketamine. ERK inhibition does not significantly affect its antidepressant-like effects (Yang et al., 2018b).
-
- CREB-BDNF Pathway: Although Esketamine activates CREB and BDNF pathways, it is not as potent as Arketamine (Yao et al., 2022).
Arketamine:
-
- ERK Signaling: Arketamine activates ERK signaling more robustly, leading to increased CREB phosphorylation and BDNF expression in microglia. This is a key mechanism for its long-lasting antidepressant effects (Yao et al., 2022).
-
- CREB-BDNF Pathway: The antidepressant effects of Arketamine are strongly linked to activating the CREB-BDNF pathway, making it more effective inducing long-term mood improvement (Yao et al., 2022).
*In humans and various chronic animal models of depression, the ERK signaling was significantly downregulated in the prefrontal cortex and hippocampus, two core areas implicated in depression. Inhibiting the ERK pathway in these areas caused depression-like behavior.
*Current data show that ERK-NRBP1-CREB-BDNF signaling in microglia might contribute to antidepressant-like effects of (R)-ketamine compared to (S)-ketamine. Therefore, it is likely that ERK-NRBP1-CREB-BDNF signaling in microglia would be a new target for depression.
Transforming growth factor β1 (TGF-β1) signaling*
Esketamine:
-
- TGF-β1 Role: There is limited evidence suggesting a major role for TGF-β1 in the antidepressant effects of esketamine. The focus has primarily been on Arketamine for TGF-β1-related effects (Zhang et al., 2020).
Arketamine:
-
- TGF-β1 Role: The antidepressant-like effects of Arketamine are significantly linked to TGF-β1 signaling. Microglial TGF-β1 contributes to its antidepressant actions, and partial microglial depletion blocks these effects (Zhang et al., 2020).
* (TGF-β1 plasma levels are reduced in Major Depressed patients (MDD), correlate with depression severity, and significantly contribute to treatment resistance in MDD.)
Opioid Receptors
Esketamine:
-
- Opioid Receptor Interaction: Esketamine interacts with mu-opioid receptors, although this interaction is less crucial for its antidepressant effects compared to NMDAR antagonism (Bonaventura et al., 2021; Zhang and Hashimoto, 2019b).
Arketamine:
-
- Opioid Receptor Interaction: Arketamine shows minimal interaction with opioid receptors compared to esketamine (Bonaventura et al., 2021; Zhang and Hashimoto, 2019b).
*(Opioid receptor subtypes, regulate mood-related processes including reward, motivation, and social behaviors. The opioid system is implicated in emotional processing, and this system is dysregulated in depression.)
Sigma-1 Receptors*
Esketamine:
-
- Sigma-1 Affinity: Esketamine has a higher affinity for sigma-1 receptors than Arketamine (Bonaventura et al., 2021). However, sigma-1 receptor involvement in antidepressant effects is less significant for Esketamine than NMDAR antagonism.
Arketamine:
-
- Sigma-1 Affinity: Arketamine has a lower affinity for sigma-1 receptors than Esketamine (Bonaventura et al., 2021). Sigma-1 receptor involvement is not a major factor in its antidepressant effects.
* (Sigma-1 receptors modulate depression by acting on dopamine neurotransmitters is widely reported. For instance, PTZ enhances the antidepressant activity of the dopamine reuptake inhibitor bupropion while the sigma-1 receptor antagonist reversed the effects)
KCNQ2 Channel*
Esketamine:
-
- KCNQ2 Role: There is limited information on the role of KCNQ2 channels in the antidepressant effects of esketamine, though it is known that KCNQ2 activation could potentially impact antidepressant-like effects (Lopez et al., 2022).
Arketamine:
-
- KCNQ2 Role: Arketamine’s sustained antidepressant-like effects might be associated with KCNQ2 channel regulation, though the specific role of KCNQ2 in its effects is still being studied (Lopez et al., 2022).
*(Preclinical studies point to the KCNQ2/3 potassium channel as a novel target for the treatment of depression and anhedonia, a reduced ability to experience pleasure.)
Summary
-
- NMDAR Blockade: Esketamine is more potent in NMDAR blockade, while arketamine is less potent.
-
- AMPAR Dependency: AMPAR activation is critical for Esketamine’s antidepressant effects; arketamine also involves AMPAR. But to a lesser extent.
-
- ERK and CREB Pathways: Arketamine activates ERK and CREB-BDNF pathways more effectively than Esketamine, contributing to its more prolonged antidepressant effects.
-
- TGF-β1 Signaling: Arketamine’s antidepressant effects are associated with TGF-β1 signaling in microglia, unlike esketamine.
-
- Opioid Receptor Interaction: Esketamine interacts with opioid receptors, whereas arketamine does not.
-
- Sigma-1 Receptors: Esketamine has a higher affinity for sigma-1 receptors than arketamine.
-
- KCNQ2 Channel: Arketamine’s role in KCNQ2 channel regulation has more data than esketamine.
Despite this ongoing conflict on whether Arketamine or Esketamine is a better therapeutical drug, the compound has made a tremendous evolution in the world of psychotherapy, including some novel treatments such as Ketamine VR, mixing Ketamine with Virtual Reality by Dr. Melissa Selinger in collaboration with Hamilton Morris, David Lobster from the Light Clinic and Dr. Tim Canty from Manhattan Restorative.
Podcast featuring Dr. Melissa Selinger.
In an experimental field, the use of Ketamine to treat post-finasteride syndrome by the molecular biologist Suat Neven.
Link to Suat Neven’s post-finasteride syndrome experiments!
As previously mentioned, as of now, studies conducted with Ketamine solemnly had two options. Either use the Ketamine nasal spray Spravato or Ketamine IV as a form of treatment (both options require a patient to stay in a clinic and be monitored for around 2 hours, thus augmenting the cost of the treatment). However, soon, we might be witnessing the development of what could be a game-changer in the world of Ketamine. A slow-releasing racemic Ketamine tablet (R-107), half R-Ketamine/S-Ketamine, that is currently being studied by Paul Glue with the University of Otago in New Zealand and sponsored by Douglas Pharmaceuticals.
In the study, 231 patients with TRD were given 120mg of R-107 per day for five days, and 168 patients were included in double-blind R-107 doses or placebo for 12 weeks. In the double-blind study, patients were given a 180mg dosage twice weekly and showed significant and clinically meaningful improvement in depressive symptoms based on the MADRS score (Montgomery–Åsberg Depression Rating Scale (MADRS) is a ten-item diagnostic questionnaire which mental health professionals use to measure the severity of depressive episodes in patients with mood disorders) compared to those with placebo, with the group of treatment difference of 6.1 in the MADRS scale.
The side effects commonly observed in clinical trials of IV or intranasal ketamine (dissociation, sedation, and increased blood pressure) were minimal. The overall tolerability was good, allowing patients to continue the double-blind phase at home after day eight, and the clinic visits were brief.
The dosage in the enrichment phase (120 mg daily for five days) was based on observations from case reports from patients with pain and TRD receiving continuous ketamine infusions for five days, who reported mood improvements occurring by 24-72 hours. The tablet formulation’s sustained exposure to norketamine over 24 hours after once-daily dosing provided a similar prolonged pharmacokinetic exposure. Additionally, during the study, participants didn’t report craving for the tablets.
Information about slow-releasing ketamine tablets (R-107) is limited at the moment. However, the data is promising and could potentially make ketamine treatments more accessible and affordable to everyone. Patients may not need to stay at the clinic after taking the compound, which could reduce the overall cost of the therapy and the administration of the compound in comparison to IV Ketamine.
Nonetheless, it’s important to note that if this medication becomes FDA-approved, it may aid future experimental and novel research as those mentioned above.


