Nitrous oxide from an Anesthetic to a party drug
From being a multiple-purpose propellant for spacecraft, a widely used tool in dental clinics, and preserving whipped cream, nitrous oxide has made its way to becoming a party drug and becoming illegal in some countries in the United Kingdom after the Psychoactive Substances Act in 2016.
The isolation of nitrous oxide and oxygen was first discovered by the English chemist Joseph Priestley in 1772 during his experiments and observations of different kinds of air.
“The diminution of common air by a mixture of nitrous air, is not so extraordinary as the diminution which nitrous air itself is subject to from a mixture of iron filings and brimstone, made into a paste with water. This mixture, as I have already observed, diminishes common air between one fifth and one fourth . . . but when it is put into a quantity of nitrous air, it diminishes it so much that no more than one fourth of the original quantity be left. The effect of this process is generally perceived in five or six hours, about which time the visible effervescence of the mixture begins; and in a very short time it advances so rapidly, that in about an hour almost the whole effect will have taken place. If it be suffered to stand a day or two longer, the air will still be diminished farther, but only a very little farther, in proportion to the first diminution. The glass jar, in which the air and this mixture have been confined, has generally been so much heated in this process, that I have not been able to touch it.”
– Joseph Priestley
The remaining air didn’t enter in combustion and had no smell, in comparison to nitric oxide. Priestley decided to name it “dephlogisticated air”, along with the discovery of this species of air, Priestley isolated what he named “Dephlogisticated nitrous air” today known as nitrous oxide.
In the following year he claimed that a mouse would live longer in his “dephlogisticated air” than in common air, he later concluded that it was better than common air and proceeded to breathe it.
“The feeling of it to my lungs was not sensibly different from that of common air; but I fancied that my breast felt peculiarly light and easy for some time afterwards. Who can tell that, in time, this pure air may become a fashionable article in luxury. Hitherto only two mice and myself have had the privilege of breathing it.”
– Joseph Priestley
Later Antoine Lavoisier, repeated Priestley’s experiments concluding that common air consisted of a mixture of two elastic fluids, one necessary for combustion and respiration while the other would support neither. Such discoveries were the bridge to the renaming of dephlogisticated air to oxygen.
It was two decades later that the effects of nitrous oxide were firstly discovered by the British chemist Humphry Davy. After being appointed Superintendent of the Medical Pneumatic Institution, set up by Thomas Baddoes.
Baddoes was the leading authority on pneumatic disease, a sector concerned with the administration of medicinal airs. His purpose was to understand the pharmacological effects of inhaling gases to treat various diseases. To achieve such results, an apparatus for producing and receiving various airs had already been designed by James Watt in collaboration with Beddoes.

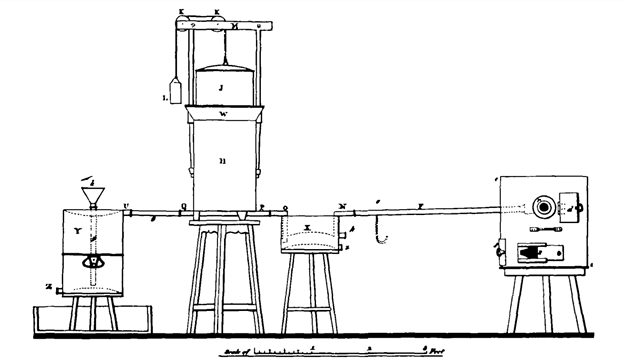
Davy then used the apparatus and set himself to investigate the physical, chemical, and biological properties of nitrous oxide. In a year he carried out numerous experiments publishing them in his book, Researches, Chemical and Philosophical; chiefly concerning nitrous oxide, or dephlogisticated nitrous air, and its respiration (Davy, 1800b). At first, his experiments started with the preparation of nitrous oxide from nitrous air, Davy then adopted an updated method to obtain his product by the thermal decomposition of dry ammonium nitrate, NH4NO3, a method firstly introduced by Bethollet in 1785. After heating the ammonium nitrate the nitrous oxide would be collected in a gasometer and stored in an air-holder, an enclosed tank filled with water. As the water was drained from the bottom of the tank, the gas would be aspirated to the top of the hydraulic bellows, which served as a reservoir. The water from the air holder was allowed to be saturated with gas and was stored in special containers.
Still in the 1800s Clayfield designed a bell–jar–upon–mercury, by using a spiral pulley, and balance was achieved. This method was used for numerous quantitative experiments, in these experiments, the “airs” would be inhaled from oiled-silk bags, and a special mouthpiece with two silk valves was designed, however, such piece didn’t seem to have many usages.
Davy then followed Priestley’s steps and confirmed many of his theories, he confirmed that 100 cubic inches of water absorbed 54 cubic inches of nitrous oxide at 46°F (approximately 7°C), and he found that 100 cubic inches of pure nitrous oxide weighed 50-1 grains at 50°F (10°C) and an atmospheric pressure of 30-7 (inches of mercury).
Unlike most of opinions, Davy believed that nitrous oxide was respirable and could support life for longer time than any other gas, except oxygen.
“Of respirable gases, or those which are capable of being taken into the lungs by voluntary efforts, one only has the power of uniformly supporting life—atmospheric air. Other gases, when respired, sooner or later produce death; but by different modes”
–Humphry Davy
His belief led to the first recreational inspiration of pure nitrous oxide in 1799, in the same year, Humphry repeated his experience in the presence of Thomas Beddoes.
“Having previously closed my nostrils and exhausted my lungs, I breathed four quarts of nitrous oxide from and into a silk bag. The first feelings were similar to those produced in the last experiment; but in less than half a minute, the respiration being continued, they diminished gradually, and were succeeded by a sensation analogous to gentle pressure on all muscles, attended by a highly pleasurable thrilling in the chest and extremities. The objects around me became dazzling and my hearing more acute. Towards the last inspirations, the thrilling ceased, the sense of muscular power became greater, and at last an irresistible propensity to action was indulged in; I recollect but indefinitely what followed; I know my motions were various and violent. . . . The next morning the recollections of the effects of the gas were indistinct, and had not remarks written immediately after the experiment recalled them to my mind, I should even have doubted their reality.”
–Humphry Davy, Thomas Beddoes.
Comproving nitrous oxide wasn’t destructive as previously thought, Davy kept experimenting with the Nitrous oxide, this time to reproduce euphoric symptoms similar to opium or alcohol. To achieve such results, Humphry entrapped himself inside an air-tight breathing box in the presence of Dr. Robert Kinglake, who would be responsible to release twenty quarts of nitrous oxide every 5 minutes as long as Humphry remained conscious.
“I felt a sense of tangible extension highly pleasurable in every limb; my visible impressions were dazzling and apparently magnified, I heard distinctly every sound in the room and was perfectly aware of my situation. By degrees as the pleasurable sensations increased, I lost all connection with external things; trains of vivid visible images rapidly passed through my mind and were connected with words in such a manner, as to produce perceptions perfectly novel. I existed in a world of newly connected and newly modified ideas. I theorised; I imagined that I made discoveries. When I was awakened from this semi-delirious trance by Dr. Kinglake, who took the bag from my mouth, indignation and pride were the first feelings produced by the sight of persons about me. My emotions were enthusiastic and sublime; and for a minute I walked round the room perfectly regardless of what was said to me. As I recovered my former state of mind, I felt an inclination to communicate the discoveries I had made during the experiment. I endeavoured to recall the ideas, they were feeble and indistinct; one collection of terms, however, presented itself; and with the most intense belief and prophetic manner, I exclaimed to Dr. Kinglake “Nothing exists but thought! The Universe is composed of impressions, ideas, pleasures and pains!”
– Humphry Davy
One century later, in 1844 Humphry’s discoveries were picked up by a dentist, Horace Wells. Pioneering the use of the gas as an anesthetic in dental procedures and to manage labor pain until today.
Regardless of its wide use, the mechanism behind the analgesic properties isn’t fully disclosed. Various options have been suggested to understand this mystery, researchers believe that the effects can be caused by the opioid receptors as the administration of the opioid reverse agonist, Naloxone, suppresses the analgesic effects of nitrous oxide. Another mechanism of action seems to be via indirect T-type calcium channel inhibition by Nitrous oxide. Further research has shown that the gas also has some action in the two-pore domain TREK-1 potassium channel, which are important in various types of pain receptors, this way being a hypothesis behind the anesthetic and analgesic actions of N2O. It’s also known that Nitrous Oxide has a major site of action in the NMDA ( N-methyl-D-aspartate) receptors, which are the natural receptors for endogenous glutamate and are excitatory in nature, this way nitrous oxide may inhibit excitatory signaling in the CNS (Central Nervous system), thus having dissociative anesthetic effects similar to Ketamine.
The popularization of the effects of Nitrous Oxide was first noticed around 2010 and keeps increasing by the day majorly as it’s cheap, widely available, and easy to obtain. Today Nitrous Oxide, going by the street name whippets, nangs, nozzies, and bulbs is the second most popular drug in the United Kingdom, as in England and Wales 8.7% of 16- to 24-year-olds had taken it, up from 6.1% in 2012/2013. Together with the data provided recently a teen was discovered driving to a party and found with 2,000 Nitrous Oxide canisters in his car thus leading to his arrest. (link: https://www.walesonline.co.uk/news/wales-news/teen-driving-party-found-2000-24255396)
This popularization lead to various robberies of hospitals to obtain Nitrous Oxide canisters, which would later be sold for recreational usage in parties, events, and festivals, sometimes creating stock ruptures in clinical facilities. This uprise, along with the issues caused in clinical settings, lead to the prohibition of the substance in the UK along with various scare stories provided by the media.

Despite the portrait made by the media, the full potential of Nitrous Oxide is yet to be explored as the gas, also known as Laughing Gas, has recently shown potential for the treatment of mental illnesses such as depression. Being an NMDA receptor antagonist, nitrous oxide just like ketamine has a rapid antidepressant effect in patients with TRD (treatment-resistant depression) these effects were sustained for at least 24 hours, and in some patients as long as one week, resulting in treatment response in 20% of patients. In comparison to Ketamine, nitrous oxide had a similar onset action (approximately 2 hours) however, it appeared to be devoid of hallucinatory effects, which may result in more favorable pharmacokinetics due to its offset that occurs in order of minutes.
Regardless of its wide use, Nitrous Oxide has its downside. As with every other substance, when the user consumes the substance recklessly the gas can become neurotoxic. Various mechanisms are responsible for N₂O neurotoxicity, being the most known being an NMDA antagonist, enzyme inhibition, and alteration of cerebral flow.
Certain brain conditions can make a patient more vulnerable to each form of toxicity. Neonatal brains are prone to NMDA antagonism. Vitamin B12 deficient patients are susceptible to homocysteine-mediated problems and lastly, patients with a damaged brain are more prone to changes in the cerebral flow, hence, using Nitrous Oxide should be done cautiously even in clinical scenario.
REFERENCES:
A HISTORY OF NITROUS OXIDE AND OXYGEN ANAESTHESIA PART I: JOSEPH PRIESTLEY TO HUMPHRY DAVY BY W. D. A. SMITH Department of Anaesthesia, Leeds University, England
Researches, Chemical and Philosophical; Chiefly Concerning Nitrous Oxide: Or Dephlogisticated Nitrous Air, and Its Respiration (Humphry Davy)
ISSN 0094-5765, https://doi.org/10.1016/S0094-5765(01)00047-9.
ISSN 0006-3223, https://doi.org/10.1016/j.biopsych.2014.11.016.
Elizabeth Rough, Jennifer Brown: Nitrous oxide: No laughing matter?
Block RI, Ghoneim MM, Kumar V, Pathak D. Psychedelic effects of a subanesthetic concentration of nitrous oxide. Anesth Prog. 1990 Nov-Dec;37(6):271-6. PMID: 2097905; PMCID: PMC2162553.
Savage S, Ma D. The neurotoxicity of nitrous oxide: the facts and “putative” mechanisms. Brain Sci. 2014 Jan 28;4(1):73-90. doi: 10.3390/brainsci4010073. PMID: 24961701; PMCID: PMC4066238.
Berkowitz B.A., Finck A.D., Ngai S.H. Nitrous oxide analgesia: Reversal by naloxone and development of tolerance. J. Pharmacol. Exp. Ther. 1977;203:539–547
Orestes P., Bojadzic D., Lee J., Leach E., Salajegheh R., Digruccio M.R., Nelson M.T., Todorovic S.M. Free radical signalling underlies inhibition of CaV3.2 T-type calcium channels by nitrous oxide in the pain pathway. J. Physiol. 2011;589:135–148
Gruss M., Bushell T.J., Bright D.P., Lieb W.R., Mathie A., Franks N.P. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol. Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443
ALAINA M. JASTER, BS : Is Nitrous Oxide a Psychedelic Drug?
van Amsterdam J, Nabben T, van den Brink W. Recreational nitrous oxide use: Prevalence and risks. Regul Toxicol Pharmacol. 2015 Dec;73(3):790-6. doi: 10.1016/j.yrtph.2015.10.017. Epub 2015 Oct 22. PMID: 26496821.
Peter Nagele M.D., M.Sc., Andreas Duma M.D., M.Sc., Michael Kopec, M.D., Marie Anne Gebara M.D., Alireza Parsoei M.D., Marie Walker M.D., Ph.D., Alvin, Janski Ph.D, Vassilis N. Panagopoulos M.D., Pilar Cristancho M.D., J. Philip Miller A.B.,Charles F. Zorumski M.D., Charles Conway M.D., Nitrous Oxide for TreatmentResistant Major Depression: a Proof-of-Concept Trial, Biological Psychiatry, http://dx.doi.org/10.1016/j.biopsych.2014.11.016