Impact of new Halogenated Tryptophan Derivatives on Psychedelic Research
A new method recently published by Reed, K.B., Brooks, S.M., Wells, J. et al. Shared groundbreaking research proving the possibility of allowing varied regioselectivity of enzymes offering halogenation across the 5-, 6-, or 7- positions of tryptophan, with the 7- position being the most studied in large-scale efforts.
The team of researchers demonstrated the first gram-scale production of 7-chloro-tryptophan from a tryptophan feed in vitro using cross-linked enzyme aggregates (CLEAs). Recent efforts in engineering Corynebacterium glutamicum demonstrated the first de novo and in vivo gram-scale production of 7-bromo-tryptophan.
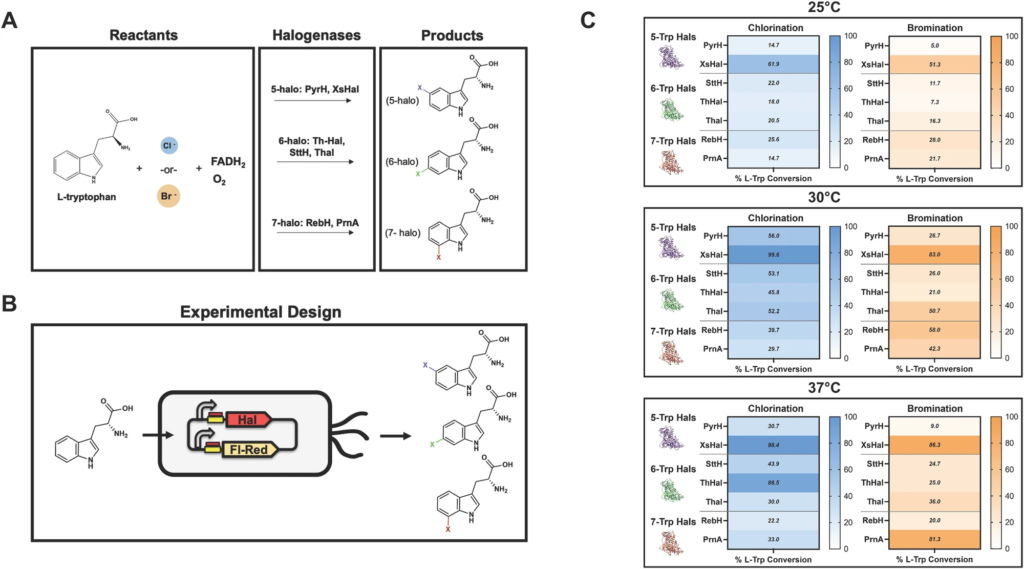
This research paves the way to expand bio-based production to alternative hosts and halogenation positions within tryptophan at a scale relevant to commercially viable industrial production.
But what is tryptophan, and how can this influence the psychedelic scene?
Tryptophan serves as a gateway to a diversity of natural products ranging from small molecules such as indole, kynurenines, quinones, and tryptamines (one of the main three chemical classes housing psychedelic compounds), to larger molecules like violacein, strictosidine, and beta-carbolines.
New-to-nature halogenated alkaloids, indigoids, and auxins have been created in planta. Additionally, halogenated quinolines and alkaloids have been achieved using yeast bioproduction platforms. While these examples demonstrate advances in halogenated metabolism, they don’t describe de novo microbial production of diverse halogenated tryptophan-derived compounds starting from simple sugar-starting materials.
Considering such conditions is essential given the relatively expensive and low-water-soluble substrate used in previous studies, such as indole, tryptophan, or other halogenated precursor molecules, as well as a need for de novo production in planta to rely on variable, seasonally-dependent crop yields.
By using a synthetic, modular co-culture system, researchers were able to generate 26 distinct halogenated molecules de novo from glucose, including new-to-nature beta carbolines, prodrug precursors to 4-chloro- and 4-bromo- kynurenine, plant hormone precursors, and other pharmaceutically relevant precursor molecules including tryptamines and indoles.

Impact on psychedelics
In the world of psychedelics, this method may integrate the birth of various tryptamine-based psychedelics and, to a broader extent, the birth of new halogenated beta-carbolines used as MAOI alternatives for pharmahuasca blends, for example, the possibilities are endless.
Enhanced Diversity of Compounds
-
- New Psychedelics: The platform enables the creation of a wide range of halogenated tryptophan derivatives. This can lead to the discovery of new psychedelic compounds with unique properties, expanding the toolkit for researchers.
-
- Structure-Activity Relationship (SAR) Studies: With a diverse library of compounds, researchers can systematically study the relationship between the chemical structure of tryptamines and their biological effects, enhancing understanding of how different modifications affect psychoactivity and therapeutic potential.
Improved Safety and Efficacy
-
- Optimized Compounds: By producing diverse derivatives, researchers can identify compounds that have an improved safety profile or enhanced therapeutic efficacy. This can lead to the development of psychedelics that have fewer side effects or are more effective at lower doses.
-
- Targeted Effects: Halogenated derivatives might interact differently with various serotonin receptors, allowing for the development of psychedelics with more specific and predictable effects, potentially reducing the risk of adverse reactions.
Scalability and Consistency
-
- Consistent Production: Synthetic biosynthesis platforms can ensure a consistent and scalable production of these compounds, which is crucial for both research and potential therapeutic use. This consistency is essential for conducting reliable scientific studies and clinical trials.
-
- Reduced Costs: Biosynthetic production can be more cost-effective than traditional chemical synthesis, especially for complex molecules. Lower costs can facilitate more extensive research and development efforts.
Ethical and Environmental Benefits
-
- Sustainable Production: Biosynthetic methods can be more environmentally friendly compared to traditional chemical synthesis, reducing the use of harmful chemicals and waste products.
-
- Ethical Considerations: By producing these compounds in a laboratory setting, the need to extract them from natural sources (which can sometimes be endangered or difficult to cultivate) is eliminated, aligning with ethical and conservationist principles.
Facilitating Regulatory Approval
-
- Regulatory Compliance: Consistent and high-purity production methods are more likely to meet regulatory standards required for clinical trials and therapeutic applications. This can accelerate the approval process for new psychedelic treatments.
-
- Standardization: Standardized production methods ensure that the compounds used in research and therapy are of known and reliable quality, which is crucial for gaining regulatory approval and for ensuring patient safety.
Expanding Therapeutic Applications
-
- Novel Therapeutics: The discovery of new halogenated tryptamines can lead to the development of novel therapeutics for mental health disorders such as depression, anxiety, PTSD, and addiction. Halogenation can alter the pharmacokinetics and pharmacodynamics of these compounds, potentially leading to improved therapeutic profiles.
-
- Personalized Medicine: The ability to produce a variety of compounds may facilitate personalized approaches to psychedelic therapy, where treatments are tailored to the specific needs and genetic makeup of individual patients.
However, we must note something very relevant to this method in psychedelic research.
There’s a constant argument regarding the safety of the 4-position in the indole ring in tryptamine-based compounds. Modifications of the 4-position in the indole ring may result in various compounds with neurotoxic profiles, for example, 4,5-dihydroxytryptamine. For this reason, we advise you to guide your research carefully.
Stay safe!
