ATOMIC PROBLEMS
The periodic table is the first thing students get to know in their chemistry class, formed by 118 elements, all of which influence our general life, and science.
Despite today’s periodic table being “complete” at this day and age, not always the periodic table was constituted of 118 elements, nor did the elements have the same name. In fact, various elements have been found in recent years, in fact, some of them were even found in the 90s and approximately 59 elements ended up switching their names.
Knowing this, few questions might rise in our minds:
“How does one create an element?”
“Who created those elements?”
“Who is responsible for naming elements and compounds?”
In order to uncover these questions these problems, a few scientific digging and historical digging are needed.
The creation of an element
In theory, the creation of an element is done by adding and removing protons. Hydrogen has one proton, Helium 2, Lithium 3 and the list goes on, however, there’s one issue with this theory, the number of neurons can vary, and this number of neurons will influence the stability of the compound.
In theory, the creation of an element is done by adding and removing protons. Hydrogen has one proton, Helium 2, Lithium 3 and the list goes on, however, there’s one issue with this theory, the number of neutrons can vary, and this number of neutrons will influence the stability of the compound.
This instability can result in isotopes such as Carbon-14, a radioactive isotope of Carbon that has an energy imbalance that it wants to rectify. For this to happen, the compound converts one of its extra neutrons into a proton, firing off a dangerous electron and a neutrino to account for the imbalance (this phenomenon is named beta-minus decay).
In this case, once this event happens, we’re left with an element with one less proton, 7 protons. Therefore, we have Nitrogen. Due to having one too many neutrons one element transformed into a different element.
The Italian Physicist Enrico Fermi understood this practice and theorized about going furthest to the edge of the periodic table, using Uranium to create a new element, by firing particles such as neutrons, protons, and alpha particles, which then fuse with the particles forming a new element (a process known as nuclear transmutation).
Once one is familiar with nuclear synthesis, one comes across a term that difficult the task of creating a new element, “Island of Stability”, a concept in nuclear physics that refers to a predicted region of the periodic table where certain elements are expected to have longer-lived, more stable isotopes.
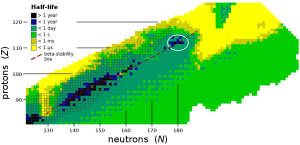
Such stability is due to the neutron to proton ration in the nucleus.
Maria Goeppert-Mayer broke the rule of adding particles to existing elements and expect to land on the “Island of Stability” by finding certain “Magic Numbers”, those being 2, 8, 20, 28, 50, 82. In element terms that’s helium, oxygen, calcium, nickel, tin, and lead.
These magic numbers are specific values of the neutron to proton ratio that result in particularly stable nuclei, and the island of stability refers to a region where these ratios are expected to be prevalent for heavy elements. The existence of this region has yet to be fully confirmed, and the creation of superheavy elements and their isotopes that lie within the predicted “island” is an active area of research in nuclear physics.
“Who created those elements?”
As previously mentioned, the Italian Physicist Fermi using his technique thought he was able to create the elements 93 and 94 for the first time, however, once further research was made into those elements it was found in the fact that Fermi was able to achieve something far more astonishing than creating a new element. He was able to make an atom EXPLODE, split it in two, a nuclear fission.
With the aid of Maria Goeppert-Mayer’s “magic numbers” various companies were set to the Element Hunt, to find as many elements as possible.
Thus, Berkeley was able to find the elements 93, 94, 95, 96, 97, 98, 99, 100, and 101.

Dubna was able to find elements 102, 103, 104, and 105.
Lastly, GSI entered the scene and found elements 107, 109, and 108.

The race was tight and with various back and forwards between them, creating massive confusion and debates on who created the compound and whether what they had created was in fact an element, hence the organization who is responsible for the nomenclature of elements and compounds would have to intervene.
“Who is responsible for naming elements and compounds?”
The entity responsible for the name of elements and compounds is widely known in the world of chemistry, and students, an organization going by the name of IUPAC (International Union of Pure and Applied Chemistry).
IUPAC was established in 1919 after Kekulé had addressed the need for an international standard for chemistry to create an international naming system for chemical elements and compounds.
Needless to say, IUPAC would have a crucial role in the Element Hunt by setting a standard rule for what could be considered an element discovery.
An element must be stable enough to exist for more than 10^-14 seconds. The necessary time for electrons to form a cloud around a nucleus. In addition, IUPAC would also be responsible to set a name for which element in order to make the nomenclature globalized.
The man who fooled the world
Fermi truly believed that he had created a new element, despite the outcome. However, one man fooled the world into believing various new elements had been created, his name is Victor Ninov.

Ninov, a physicist himself, developed one software that would both create a ruckus in the Element Hunt and be a blessing in 1988. Goosy, the software created by Ninov was responsible to analyze chains of radioactive samples, whereas, previously that task would need to be done manually.
First, the software used by the German lab GSI, while in strains with GSI Ninov was able to add important bullet points to his resume, as he was co-discoverer of three new elements, 110, 111, and 112.
Knowing this, Berkeley decided to hire Ninov, putting him in charge of the data analysis, he was put on the team of the Polish physicist Robert Smolanczuk, who had the theory to skip everything from the elements 113 to 117 and aim for 118. He planned to fire Krypton-86 at a lead-208 target.
Ninov backed up his plan and drew a diagram with three perfect alpha decay chains that ended at element 106. Thus, it would be fair to say that the team had discovered not just 118, but 116 and 114.
Eager to catch up with Berkeley, GSI decided to repeat Smolanczuk’s theory yet they weren’t able to see the alpha decay chain, recorded by Ninov, similarly to GSI’s experience, teams in France and Japan weren’t able to obtain the results either.
Ninov became reluctant to talk about his research and continuously deflected questions about his discovery, Berkeley decided to re-run their experiment, this time with an independent team, in 2000 and weren’t able to reproduce the previous result either.
Berkeley decided to take apart their detector system and clamps down on their procedures and even considered whether their beam was made of Krypton, and wasn’t contaminated with another element.
In 2001, they were ready to begin testing again with the new setup, one month later they found what they were looking for, one single alpha decay chain, once again detected by Victor Ninov.
At first, Ninov was the only person who was familiar with GOOSY, but as years passed, many others became familiar with the interface and didn’t get the same data as Ninov, no one could seem to find the element 118.
In June 2001, Darleane Hoffman, assembled a team to analyze every bit of data regarding element 118, they went through every file. Based on their findings the group decided to do an independent review committee, which had the official name of “The Committee for the Formal Investigation of Alleged Scientific Misconduct.”
Once the investigation took place, it was found that the log files had clear evidence of tampering, and so did the logs from 1999, the energy values, and time values were altered and some events were entirely added. The values had been tweaked enough to suggest alpha decay chains, and the entire fundamental basis of the paper was made up. The committee later found that every tweaked log file belonged to the same user account VNinov.
On November 21st, 2001, Ninov was placed on administrative leave and was officially fired in May 2002 for misconduct.
Lost elements
With the existence of IUPAC, plenty of names of existing compounds have been rejected, some generated controversies and some of them had minor changes, and some had a complete change in their nomenclature.
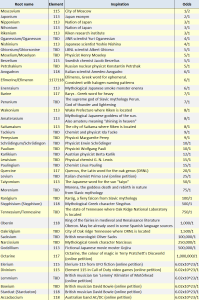
Below will be presented a list based on the book by Marco Fontani “The Lost Elements: The Periodic Table’s Shadow Side”
Element 4: Glucinium (Beryllium)
Firstly reported by the French chemist Louis Nicolas Vauquelin while examining both emerald and beryl in 1798. Vauquelin reported that both minerals would have a new element, he named this newfound element Glucine, with the symbol Gl, due to the similarity to the amino acid glycine, this name was criticized. Once the element was isolated in water in 1828, the acceptance of the name beryllium was suggested by the chemist Martin Henrich Klaproth. In 1949, IUPAC ruled that the element should only be called Beryllium.
Element 5: Boracium (Boron)
Boron was isolated simultanously by threw chemists during the same period, the French chemists Louis-Joseph Gay-Lussac and Louis-Jacques Thénard and the English chemist Sir Humphrey Davy in 1808. Davy proppsed the name of the element to be Boracium, which eventually was changed to Boron.
Element 7: Azote (Nitrogen)
Antoine Lavoisier discovered element 7 in 1776, the element was latee proposed the name Azote. Chemists around the world weren’t quite pleased with the name, hence the element was renamed to Nitrogen.
Element 9: Fluore (Fluorine)
André-Marie Ampère, proposed that hydrofluoric acid, like hydrochloric acid was a binary compound consisting of hydrogen and another element. Thus, he proposed the name of fluor or phtore for this element, but left the choice to Sir Humphrey Davy, whom he had corresponded on the subjet. This eventually became the element’s current name, Fluorine, the element would later be isolated in 1886.
Element 10: Novum (Neon)
Sir William Ramsey discovered all the other elements in group 18 of the periodic table, in 1898, he discovered Neon and decided to use the element’s name the suggestiom of his son “Novum”. However, Ramsay wanted the name to be derived from the Greek like his previous discoveries, for this reason he made a slight modification to the nomenclature of the name, giving birth to the name Neon.
Element 12: Magnium (Magnesium)
In 1808, Humphrey Davy isolated magnesium and named it Magnium, Davy prefered the name rather than magnesium after the oxide from magnesia alba, as he didn’t want the name to be confused the element manganese. However, the name presisted, although magnium is still used in certain countries. In another instance, Talcinium was suggested in 1838, however this nomenclature didn’t gain any track and was never used.
Element 21: Gadenium (Scandium)
In 1886, Alexander Pringle believed he had found four new elements, he named them polymnestum, erebodium, gadenium and hesperisium. However, his determinatiom of the atomic weights of these elements was poor, thus he failed to describe any new elements. It’s likely that the gadenium was the already discovered scandium and the other elements were incorrect deductions by Pringle and most likely mixtures of already known elements.
Element 22: Menachite (Titanium)
William Gregor discovered Titanium and gave it the name menachite after the black sand he found in 1789. In 1794 Martin Henrich Klaproth found the same element and named it titanium, Klaproth didn’t notice this until three years later. Despite Gregor’s priority the name Titanoum stuck and menachite was forgotten.
Element 23: Panchromium (Vanadium)
Andrés Manuel de Río, first found element 23 in 1801, he decided to name the element panchromium, due to the various schemes of colors exhibitted in its salts. He later switched the name to erythronium, Andrés’s discovery of a new element was erroneously challanged and he retracted his discovery. This element would later be rediscovered by the Swedish chemist Nils Gabriel Sefström, who chose the name Vanadium to the element, confirming De Rio’s discovery.
The Italian minerologist Arcangelo Scacchi, would also claim to have discovered a new element in the crusts left by the erruption of Mount Vesuvius two centuries earlier, after analysis this new element turned out to be Vanadium.
Element 32: Neptunium (Germanium)
To anyone who is familiar with the periodic table knows that Neptunium has a place in this famous table, however this element is element 93, not 32.
Element 32 was first isolated by the German chemist Clemens A. Winkler in 1886, and originally named it Neptunium. He soon realized that the namr had beem used for the erroneous claimed discovery of a new element by John Herschel, in order to reduce confusion in between these two elements Winkler named his element Germanium instead.
Element 33: Polymnestum (Arsenic)
Polymnestum was the name given by other erroneously claimed new elements by Alexander Pringle, this new element proposed atomic weight and a description that corresponds closely to the already known element Arsenic.
Element 34: Hesperisium (Selenium)
Another Pringle’s failed element, the description given of hesperisium, corresponds to that of Selenium, which was known at the time.
Element 35: Muride (Bromine)
Element 35 was discovered by Antoine-Jérôme Balard who suggested the name Muride, the name was later changed by the French Academy of Sciences who proposed the name Brome, which eventually became Bromine
Element 36: Eosium (Krypton)
Element 36 is another noble gas found by William Ramsey, a friend who also worked on the discovery of the new element Marcellin Berthelot, suggested the name Eosium, Greek for “dawn”, Ramsey didn’t agree to this nomenclature and named the element Krypton.
Element 39: Lucium (Yttrium)
In 1896, a new element under the name Lucium was supposedly discovered by the French chemist Prosper Barrière, after analysis, the element turned out to be impure Yttrium. This was the first article of Barrière that was endorsed by several chemsits at the time who later denied their involvement.
Element 41: Columbium (Niobium)
In 1801, the English chemist Charles Hatchett, claimed the discovery of a new element and gave it the name Columbium. One year later Tantalum was discovered and it was thought that Tantalum and Columbium were one at the same time. However, the chemist Heinrich Rose, suggested that thr mineral in which Columbium was found might contained Tantalum and other two elements (Niobium and Pelopium). Columbium and Niobium were later found to be the same element, not Columbium and Tantalum.
Such confusion reflected in the periodic tables at the time, Columbium and Niobium were used to refer to element 41 roughly until 1949, when IUPAC ruled that Niobium should be the official name of the element.
Element 42: Erebodium (Molybdenum)
Yet another of Alexander Pringle’s failed elements, which corresponded to the already known Molybdenum.
Element 43: Masurium (Technetium)
Element 43 doesn’t naturally occur outside uranium deposits, where it’s created in trace amounts as a byproduct of spontaneous fission, before it decays.
The German chemists Walter & Ida Noddack claimed the discovery of element 43 in 1925, the same time they discovered rhenium and named it Masurium. Various chemists doubted the discovery of the element and it was never corroborated by independent experiments. The element was later on created artificially by Carlo Perrier and Emilio Segrè who named it Technetium.
Element 43’s naming history doesn’t stop there though – it also has links with rhenium, as we’ll discover when we evaluate the history of element 75’s name.
Element 44: Polinium (Ruthenium)
Element 44 was discovered by Karl Ernst Klaus in 1844. However the name of the element dates back to 1828, when the name Polinium was proposed by Gottfried Wilhelm Osann who claimed to had discovered the element during that year. Nonetheless, it is thought that Osann’s discovery was merely impure iridium, Osann himself would later admit it, scuppering his claim.
Element 46: Ceresium (Palladium)
Palladium was discovered in 1803 by William John Hyde Wollaston, who originally proposed the name Ceresium, this nomenclature wouldn’t be used due to the simillarities of Cerium which was proposed for the first of lanthanide elements. Before he published his results, he changed name to Palladium instead.
Element 48: Melinum (Cadmium)
Element 48 was discovered simultaneously by two chemists Friedrich Stromeyer and Karl Hermann. Later another chemist Karl Karsten thought he had discovered a new element in zinc deposits naming it Melinum, which was found to be in fact Cadmium.
Element 52: Pilsum (Tellurium)
Element 52 was originally discovered by the Hungarian scientist Ferenc Müller von Reichenstein, who gave it the name “metallicum problematicum. It was later rediscovered by Pál Kitaibel, who named it Pilsum, and isolated by Martin Heinrich Klaproth in 1798 who gave it the name Tellurium, which remained until today.
Element 56: Plutonium (Barium)
Edward Daniel Clarke claimed to find element 56, proposing the name Plutonium, which was rejected by chemists who also isolated the element and prefered the nomenclature Barium. Plutonium would then make it to the periodic table as element 94.
Element 58: Ochroite (Cerium)
Martin Heinrich Klaproth discovered element 58 and proposed the name ochroite. At the same time, Jöns Jakob Berzelius also discovered the same element and won the nomenclature war, giving it the official name cerium.
Element 60: Didymium (Neodymium)
Neodymium and praseodymium (element 61) were once mistaken to be only one element didymium. Forty years later its dual nature was realized.
Element 61: Florentium (Promethium)
Luigi Rolla and Lorenzo Fernandes claimed to have found the element 61 Florentium after the Italian city Firenze (Florence), however, their findings were later shown to be erroneous.
Element 62: Decipium (Samarium)
Element 62 was named Decipium by the chemist Marc Delafontaine in 1878. Delafontaine would later realize that he had a mixture of elements including element 62.
A year later Paul Émile Lecoq de Boisbaudran, had isolated the element’s oxide and named it Samarium. The mineral form would, later on, be found by the Russian mining engineer Vasili Samarsky-Bykhovets making him the first person to find the pure element and naming it after them.
Element 67: Philippium (Holmium)
Delafontaine would also find element 67 in the same year, and named it Philippium. The element was later rediscovered and named holmium, which already had a common usage by the time Delafontaine’s name was established.
Element 70: Aldebaranium (Ytterbium)
Both elements 70 and 71 were discovered around the same time by two different chemists, Frenchman Georges Urbain, and Austrian Carl Auer von Welsbach, Welsbach decided the name of element 70 to be Aldebaranium, however, Urbain was credited with the discovery of both elements and choose the name neb-ytterbium (changed for the simple form ytterbium).
Element 71: Cassiopeium (Lutetium)
Welsbach chose the name lutetium was cassiopeium. Once again Urbain’s name selection was picked, and the name Lutecium was chosen, later changed to present-day Lutetium.
Element 72: Celtium (Hafnium)
The discovery of Celtium was claimed by Georges Urbain, he later realized that his claim was erroneous. Regradless, there was a debate over this name, IUPAC would then rule in favor of the name Hafnium to this element in 1930.
Element 74: Scheelium (Tungsten)
Martin Heinrich Klaproth gave the name Scheelium to element 74 as a way to honor Carl Wilhelm Scheele, who first identified the element in mineral deposits. Unfortunately, this name was then rejected.
Element 75: Nipponium (Rhenium)
The history behind Rhenium gets slightly confusing, the element was officially discovered and named by Walter and Ida Noddack in Germany, however, the compound might had been found by Masataka Ogawa in Japan previously. Masataka thought he had discovered element 43, and proposed the nae nipponium after his country, unfortunately his claims weren’t confirmed to be element 43, but in fact element 75.
Element 85: Anglo-Helvetium (Astatine)
Element 85 was filled with various false claims to be discovered, thus, has various names that didn’t end up being official from dor, viennium, alabamine, leptine, and lastly Anglo-Helvetium, the last name being proposed by the Swiss Walter Minder and the English Alice Leigh-Smith. The name was birthed from their collaboration, however when their experiments were replicated the element couldn’t be found.
Element 86: Niton (Radon)
In 1912, element 86 was named Niton by Sir William Ramsay, this name was accepted by the International Committee of Atomic Weights. The name would later be changed to Radon by Friedrich Ernst Dorn.
Element 87: Catium (Francium)
Alkalinium, Russium, Virginium, Moldavium, and Catium were names proposed for what today is Francium.
Catium being the most famous amongst those was proposed by Marguerite Perey, the physicist who discovered the element. The nomenclature was denied by one of her supervisors, the daughter of Marie Curie, Irène Joliot-Curie who believed the name would portray the image of a cat to English speakers, instead of the intended image of a “cation” Perey would then suggest Francium which was accepted until this day.
Element 88: Masrium (Radium)
Chemists Henry Droop Richmond and Hussein Off claimed to found the element in a mineral found in Egypt, and gave the name Masrium, after Egypt. The atomic weight they reported would be the same as Radium, unknown at the time. However, there is no doubt that the analysis made was flawed, and they didn’t discover a new element.
Element 89: Emanium (Actinium)
Element 89 was discovered twice by two different people, once in 1899 by André-Louis Debierne who named it Actinium, and by Friedrich Oskar Giesel in 1902 who named it Emanium. As Debierne discovered it first, the name Actinium stuck.
Element 91: Brevium (Protactinium)
In 1813, an isotope of Protactinium was found by Kasimir Fajans and Oswald Helmuth Göhring who named it Brevium due to its short half-life. In 1917 Lise Meitner and Otto Hahn discovered a more stable isotope and named it Protactinium, a name that would stick until today.
Element 92: Klaprothium (Uranium)
Uranium was first isolated by Martin Friedrich Klaproth who named it Uranit, after he recently discovered the planet Uranus, and later changed it to Uranium.
At some point, it was discussed that the element should be named Kaprothium after its discovered, but this suggestion was rejected.
Element 93: Ausonium (Neptunium)
Enrico Fermi once claimed to have found the elements 93 and 94 in 1934, he proposed the names Ausonium for element 93 and Hesperium for 94, both based on ancient names for Italy. A few days later it was debunked that Fermi didn’t create those new elements but created nuclear fission. Element 93 was later produced in 1940 and named Neptunium.
Element 94: Extremium (Plutonium)
Extremium was one of the names considered for element 94. However, the name Plutonium settled to continue the series of planet-based element names.
Element 95: Pandemonium (Americium)
Both elements 95 and 96 were constantly referred to as Pandemonium and Delirium by Glenn Seaborg and his colleague Tom Morgan. At the time, they thought of submitting both names to IUPAC. Unfortunately, at the time various suggestions had been placed. Seaborg eventually named element 95 Americium.
Element 96: Bastardium (Curium)
Another Seaborg suggestion for element 96 was Bastardium, alluding to the mythical tale of pluto’s rape of Persephone. As expected, the name wasn’t selected as the official name for the element.
Element 97: Mendelevium (Berkelium)
Element 97, had already been discovered in 1949 in Berkeley, shortly after the Soviet Union also claimed the discovery of the element naming it Mendelevium. Their claim was rejected, however, the name Mendelevium would be used to name element 101.
Element 98: Cyclonium (Californium)
Cyclonium was proposed for the element 98 due to the cyclotrons used to create superheavy elements, the name wouldn’t become official and the name Californium was then picked as an alternative.
Element 99: Losalium (Einsteinium)
Losalium was the name proposed for Element 99 by one of the teams in Los Alamos who discovered the element, the name of the element would later be changed to Einsteinium by Glenn Seaborg.
Element 100: Phoenicium (Fermium)
Phoenicium was the name proposed by the scientists at Argonne National Laboratory, the name would be rejected and the official name Fermium would remain until today. Other suggestions would be Centurium, Uclasium, and Arconium.
Element 102: Joliotium (Nobelium)
Element 102 was part of a race between Sweden, the USA, and Russia to find who had discovered it first. Nowadays it is known that the Russian team had priority and is known as the official discoverers of the element.
The team proposed the name Joliotium, and the Swedish team proposed the name Nobelium. The results obtained by the Swedish team couldn’t be replicated and they retracted their discovery, however, IUPAC instantly accepted their name suggestion, the name was again updated to Nobelium in 1994 and remained until today.
Element 104: Kurchatovium (Rutherfordium)
Element 104 was also discovered by both the US and Russia, Russians named the compound Kurchatovium after their former head of nuclear research. However, Americans won their claim for priority and named the element Rutherfordium.
Element 105: Nielsbohrium (Dubnium)
Yet another dispute between the US and Russia.
Russian scientists proposed the name Nielsbohrium after the Danish scientist Niels Bohr and the US proposed the name hahnium after chemist Otto Hahn.
This debate wasn’t over until 1997 when Dubnium was agreed upon as the official name after Dubna in Russia.
Element 106: Alvarezium (Seaborgium)
Alberto Ghiorso, who was in charge of research on new elements at Berkeley, decided to name the element Alvarezium, after physicist Luis Walter Alvarez. However, his team wasn’t prone to name the element Alvarezium and wanted to name it Seaborgium after Glenn Seaborg.
Due to an IUPAC rule, an element cannot be named after living people, as Seaborg was still alive at the time. IUPAC thus decided that the official name of the element would be Rutherfordium. This decision didn’t go well with the American Chemical Society, who complained until IUPAC reconsidered this decision. Thus the name Rutherfordium was then attributed to element 104.
Element 109: Hahnium (Meitnerium)
Another disputed element, Russian scientists suggested the name Hahnium after the chemist Otto Hahn. IUPAC would decide the name of the element to be Meitnerium after Lise Meitner.
Element 114: Russium (Flerovium)
Element 114 was suggested to be named Russium but was rejected due to it having previously been suggested for the false claim of discovering element 43. IUPAC would later decide to name the element Flerovium, a decision that confused many as the name Flerovium was also suggested and rejected for element 102.
Element 116: Leosium (Livermorium)
Leosium would have the same problem as element 114, the name had been suggested for element 43 after an unconfirmed discovery. For that reason, IUPAC stated that it could not be used again. Element 116 would later be named Livermorium after the lab that discovered the element.
Elements 113, 115, 117 and 118
To this date, elements 113, 115, 117, and 118 have received various suggestions of names, however, as of today we don’t have an actual official name approved by IUPAC.
References:
Superheavy by Kit Chapman
The Transuranium People by Glenn Seaborg, Al Ghiorso and Darleane Hoffman
When Science Goes Wrong: Twelve Tales from the Dark Side of Discovery by Simon Levay
Committee on the Formal Investigation of Alleged Scientific Misconduct by LBNL Staff Scientist Dr. Victor Ninov
The Lost Elements: The Periodic Table’s Shadow Side